Don’t Bury Your Problems
When Dawson Public Power District in Lexington, Nebraska, U.S., observed accelerated corrosion on several of its galvanized steel utility poles, the utility conducted a field and laboratory study to determine the cause of the corrosion. The study results identified the root causes of the corrosion as soil corrosivity and galvanic action attributable to copper grounding.
Galvanized steel is one of the most often specified materials for the manufacturing of poles, lattice towers and other commonly used T&D assets. This is especially true for high-voltage transmission line and substation structures.
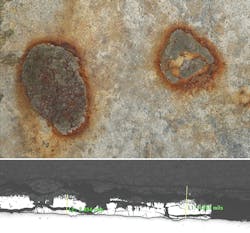
Aging galvanized steel is prone to failure because of the accelerated corrosion it experiences in corrosive soils. Dawson Power used several techniques to inspect its galvanized steel utility poles: excavation and photographic documentation, detailed electrochemical-potential field measurements, corroded galvanized steel metallurgical characterization, determination of the galvanized steel poles’ corrosion rate, continuity testing and laboratory soil characterization.
Enhanced corrosion effects from soil characteristics depend on low soil resistivity, the presence of corrosive ions and on the impingement of water tables — and other sources of moisture — on the embedded steel structure. A light microscopic and scanning electron microscopic-energy dispersive spectroscopic (SEM-EDS) analysis revealed 11 structures with the worst corrosion still had a galvanized coating protecting the underlying steel substrate. This means not all zinc was corroded and there was some protection present.
Service Life
Several factors affect the service life of buried steel utility pole structures:
- Service environment, including soil type and water table corrosivity
- External influences, including grounding effects, stray corrosion currents and weather factors
- Age of a structure
- Presence or absence of coating and cathodic protection.
Some direct-embedded steel poles and towers corrode quickly as a result of natural and man-made environmental effects. This is primarily because of corrosive soils and galvanic action, or dissimilar metal corrosion.
Field inspections and studies have led to some interesting observations about corrosion activity. First, the copper used as grounding at substations can corrode. This is a serious safety issue. Galvanized poles and galvanized anchors corrode through galvanic effects. Shield wires make the lines electrically continuous.
A balanced state that prevents corrosion can be induced by cathodic protection controlled by a rectifier. To inhibit corrosion to the greatest extent, cathodic protection should be deployed the full length of lines from substation to substation.
Through its investigation, Dawson Power came to the following conclusions:
- Galvanized anchors and poles exhibit corrosion due to soil corrosivity, copper grounding and stray currents.
- Soils and water tables are corrosive and, in certain locations, may induce extreme accelerated corrosion.
- The copper grounding at substations in corrosive soils adds to the corrosion potential of affected structures and reduces their life expectancy.
- A systemwide cathodic protection system can eliminate the adverse effects of corrosive soil and copper grounding on protected structures and add relatively maintenance-free service life to a protected line.
Corrosion Protection
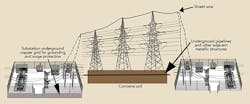
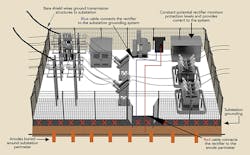
Dawson Power’s approach to protecting its galvanized steel utility pole structures involved implementing a systemwide level of corrosion protection while emphasizing safety and the protection of assets at minimum cost. This approach was founded on the utility’s ability to monitor all performance parameters in real time, by wireless telemetry and by providing access to collected data through the Internet. The solution for protecting the galvanized steel utility T&D structures at the substation level includes neutralizing the effect of copper while affording corrosion protection to the poles, anchors and copper grounding.
The innovative cathodic protection system can be summarized as follows:
- A buried anode perimeter was established around the substation.
- The buried anode perimeter was electrically connected to the buried substation copper ground grid.
- An uninsulated overhead ground wire was continuously attached to each steel pole from one substation to the next.
- Separate cables connected the rectifier to both the substation grounding system and the anode perimeter.
- The rectifier measured differences in potential and impressed a balancing current into the system.
It is important to realize the criteria for cathodic protection differs for new and aging structures. This aspect is often not considered; therefore, potential measurements can be misinterpreted after the installation of a cathodic protection system. The zinc and intermetallic layers of galvanized steel exhibit an active potential compared to carbon steel, and very high negative potentials (>-1.2 V) induced by cathodic protection may corrode the zinc layer on brand-new galvanized steel.
Another important factor for protection is the bare surface area. The cathodic protection system can protect a full line for many years in corrosive soils if the structures are fully or partially coated, or if additional ground beds are placed in between substations.
Protection Trials
Dawson Power conducted cathodic protection trials at three substations. Initial work included conducting potential surveys at the substations and at the poles between substations. This included both native and polarized potentials. Polarization methods were used to analyze the effect of cathodic protection on the reduction of corrosion current and increase in life expectancy of the galvanized poles in corrosive soil.
Wireless corrosion reference electrodes were used to monitor the cathodic protection system. An important aspect of this project is that Dawson Power was among the first in the electric utility industry to implement wireless corrosion monitoring. The system collects and analyzes corrosion data from sensors or cathodic protection equipment at the site, and automatically passes that data to a web data center. The information is converted into alert messages, indicating changes of conditions at the site, along with regularly scheduled measurement data for archiving systemwide cathodic protection system performance. As a result, users may access historical corrosion information and view graphical displays of corrosion activity.
Based on results from its trials, Dawson Power has seen a substantial increase in the remaining life of its galvanized structures because of cathodic protection. If the poles are not coated by organic coating, additional cathodic protection may be required in between substations to provide full protection on poles distant from substations.
Acknowledgments
The authors would like to thank Dawson Power for sponsoring this project and thank Matco’s engineers and technicians who assisted during different phases of this project, including Kevin Groll, Geoff Rhodes, Heather Groll, Dr. Zee and Debra Riley.
Scott Fagot ([email protected]) is manager of purchasing and facilities with the Dawson Public Power District in Nebraska, where he has been employed since 1974. Fagot spent 15 years in the field and has served as a department manager for 26 years. He is a member of the Institute for Supply Management.
Mehrooz Zamanzadeh ([email protected]) is Matco’s primary failure analyst/corrosion investigator. He is a NACE-certified specialist in corrosion, coatings, materials selection and design, and cathodic protection. Among his credits are a variety of awards, including the NACE Fellow Award, ASM Fellow Award and Colonel Cox Award. Zamanzadeh has performed corrosion risk assessment and failure analyses investigations involving corrosion, coatings and materials damage in the construction, oil and gas industries, and on aging utility and aircraft components.
George T. Bayer ([email protected]) is a technical manager at Matco. He has been with the company for 12 years and has more than 25 years of experience in a wide range of technical areas, including the characterization and testing of metallic and nonmetallic materials and coatings, development of metallic coatings, corrosion and coating failure analysis investigations, environmental exposure testing and project management.
Mentioned in this article:
Dawson Power | www.dawsonpower.com
Matco Services Inc. | www.matcoinc.com
Sidebar: Cathodic Protection
Cathodic protection is a method in which a sufficient amount of electrical direct current (DC) is continuously supplied to a submerged or buried metallic structure to mitigate, slow down or temporarily stop the natural corrosion processes from occurring. Cathodic protection systems pump electrons into the structure thus protecting them.
There are two methods for supplying DC to protect a structure cathodically: a galvanic or sacrificial anode cathodic protection system, and an impressed current cathodic protection system. The designs are based on an empirical model that may consider current and potential distributions. Wrong currents and anode positions may lead to unprotected or underprotected areas. Optimization methods combined with the boundary elements technique have become a useful tool to analyze such situations. The following items should be considered in the analysis:
- Concrete encasement cannot be ignored with regard to the mixed potential that results in varying current demand.
- Mill scale-coated structural steel (because of corrosion) under the lattice can be anticipated, as this may be proportionally higher since the mill scale is even more electropositive than copper grounding.
- On a belowgrade structure that is galvanized, not coated, the potential difference between the mill scale and zinc is higher.
- Many structures do not have a protective coating at all.
- For high-resistance soils, only certain models are likely amenable to effective sacrificial cathodic protection design.
- Protection criterion for galvanized steel is different from that of carbon steel.
Structure geometry, soil properties, environmental parameters and structure coating are salient factors that should be included in any cathodic protection design tool.