Ice accumulation is a common problem in parts of the world with cold and temperate climates. Ice buildup can be problematic on many structures including aircraft, bridges, and roads. Surfaces with poor hydrophobicity and high thermal conductivity, such as glass and metal, are especially prone to ice adhesion and buildup, including aluminum strand high voltage (HV) powerlines (figure 1). When overwhelmed with the weight of ice, the HV lines and towers can collapse, resulting in severe economic losses and in extreme circumstances, even the loss of life. Materials and structures with low ice adhesion are therefore desired, as surfaces with excellent deicing properties, or “icephobicity”, allow for easy ice removal.
Unfortunately, making surfaces “icephobic” is not straightforward, as the process of ice accretion and buildup is complex. Ice seldom coats everything with an equal thickness and rarely happens instantaneously due to super cooled rain or snow. Ice also freezes and accumulates on different surfaces at different rates. Compounding this, ice nucleation itself is still poorly understood. How much ice buildup occurs on a surface depends on a multitude of factors, including the physical characteristics of individual surfaces and objects as well as their respective temperatures. Elevated metal structures such as high voltage conductors and power lines have the greatest chances of icing due to their corresponding lower temperatures and material properties. The metallic nature of high-voltage structures is a particular detriment as these materials combine two favorable properties for ice accumulation: high surface energy and thermal conductivity, both of which encourage ice accumulation. The high surface energies of metals encourage water droplets to spread out, increasing their contact to the cold surface and accelerating freezing.
In contrast, a structure or material such as a polymer, will likely ice over more slowly. Additionally, the freezing of water droplets after they impact a surface is not immediate. The time it takes for the water droplet to freeze is dependent on several factors, including the temperature of the water droplet, its size, surface area, and the temperature of the surface which it impacts. The delay in freezing can even occur if the water droplet is super-cooled to temperatures below 0˚C. This creates an additional problem: instead of a spherical droplet freezing in place, a trail of water with a much larger surface area freezes instead. Subsequent water droplets therefore nucleate more quickly when they come in contact with ice, which only compounds the issue. Unlike snow which builds up vertically and clusters, freezing rain quickly coats and adheres to surfaces due to the viscous nature of water, making it difficult or sometimes even impossible to remove.
The misconceptions about ice accretion can often misdirect studies assessing material icephobicity, as the complex mechanisms required for ice nucleation and buildup are multifaceted and depend on both meteorological and material conditions. Testing materials for their ice adhesion strength is fraught with errors. The most direct approach is testing the interface between the ice and substrate by encasing the water during freezing in a glass or plastic cuvette mold and measuring the force to remove it. This method, however, does not account for problems caused by the mold, as well as the complex factors involved in the icing of structures due to freezing rain, which often results in uneven and unpredictable distributions of ice.
When it comes to icing of HV structures, identifying all the conditions required for ice buildup to occur is difficult, but required in order to fully understand and ultimately prevent icing. The National Science Foundation Industry/University Cooperative Research Center for Novel High Voltage/Temperature Materials and Structures (HVT Center) at the University of Denver is innovating new methods to assess ice adhesion, and developing new approaches to simulate ice accumulation due to freezing rain. The Center has already developed novel polymeric materials to reduce ice accumulation by 50%, and is currently working on even more advanced composites to eliminate ice growth altogether.
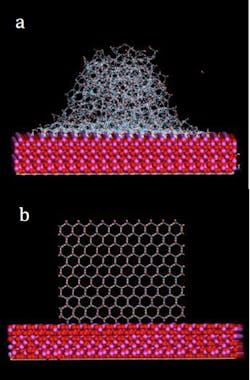
Using molecular dynamics, 3D physics models, and experimental methods, the Center has just developed new biomimetic materials to inhibit ice nucleation at the molecular level, before icing of structures or ice buildup can even occur. These composites, based upon the Antarctic Cod, mimic the cod’s anti-freeze protein, which prevents ice buildup in its veins in the frigid Antarctic waters.
This research, along with the results of many other industry sponsored and approved projects, is being presented at the HVT Center semi-annual meeting on Dec. 3-5, 2018. If you would like to learn more, please visit hvtcenter.org or contact Dr. Maciej Kumosa at [email protected].
The National Science Foundation Industry/University Cooperative Research Center for Novel High Voltage/High Temperature Materials and Structures is a T&D World media partner. For more media partners, see our complete listing.
References
1. Jones, K. F. Ice accretion in freezing rain. No. CRREL-96-2. Cold Regions Research And Engineering Lab Hanover NH, 1996.
2. Jones, K.F. "A simple model for freezing rain ice loads." Atmospheric research 46.1-2 (1998): 87-97.
3. Work, Andrew, and Yongsheng Lian. "A critical review of the measurement of ice adhesion to solid substrates." Progress in Aerospace Sciences (2018).
4. Bleszynski, Monika. "Nanoengineering of Next Generation Silicone Rubber Materials for Extreme Applications." PhD Dissertation, University of Denver (2018).
5. Woll, Theodore R. "Ice Adhesion Analysis of Severely Aged PDMS Rubbers." MS Thesis, University of Denver (2018).