Worldwide need for electricity often necessitates that high voltage (HV) systems be located in some of the most unforgiving and detrimental environments, including near or even in seawater [1-2]. Modern HV systems commonly utilize silicone rubber (SIR) based non-ceramic insulators (NCIs), which are typically resilient in normal environments. Near coastlines, however, these insulators can experience a dramatic loss of hydrophobicity caused by salt contamination (Figure 2: a-c). Without their hydrophobic properties, these polymers can rapidly degrade, leading to both electrical and mechanical failures of energized HV transmission lines [2].
Contact angles of RTV-1
It has long been established that ocean and salt-water environments are particularly damaging to polymer materials, and NCIs are no exception [1-6], but what exactly is at the root of the damage caused by aqueous salt to polymers? Chlorine has commonly been identified as the main culprit, because of its notoriously volatile nature. The real answer, however, is more complicated than that.
The National Science Foundation Industry/University Cooperative Research Center for Novel High Voltage/Temperature Materials and Structures (HVT Center) at the University of Denver has recently discovered an entirely new source of aging of NCIs in coastal environments, which could be triggered by the leakage currents found on transmission lines. The unique combination of salt, moisture, and voltage on in-service HV insulators can cause electrolysis, resulting in the formation of highly damaging compounds such as hypochlorous acid (HOCl). These compounds can rapidly degrade the NCIs by attacking the polymer chains and methyl groups of silicone rubbers. Without its methyl groups intact, the polymer rapidly loses hydrophobicity, leading to damage of the NCI, water ingress, and ultimately mechanical failure [3-7].
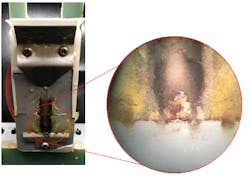
HOCl does not just cause damage on the polymer surface; it can also rapidly diffuse and cause damage from within (Figure 3: a, b). Room Temperature Vulcanized (RTV-1) SIR (used as caulking materials in NCIs) exposed to HOCl at room temperature for just a few weeks develops cavities and holes in the polymer interior, due to the rapid diffusion of HOCl. The rubber also loses its hydrophobicity twice as quickly compared with the same material submerged in ocean concentration salt water (Figure 2: b, c).
Interior cross-section of RTV-1
Further research demonstrates just how damaging oxidative compounds like HOCl can be to SIRs. RTV-2 SIR used for NCI weathersheds and housings immersed for just 24 hours in an electrolyzed salt have their resistance to dry band arcing (DBA) halved. Insulator skin from a major manufacturer can typically withstand constant DBA at 4.5 kV for more than 12 hours, with no surface damage. However, the same insulator skin, after immersion in electrolyzed salt, is only able to sustain four hours of DBA before the integrity of the material is seriously compromised (Figure 4).
Aged HV SIR insulator skin
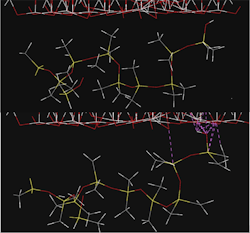
So if coastal environments cause so much damage to NCIs, how can it be prevented? One method is to include additives to the SIR formulation in order to improve hydrophobicity. Our research shows that a small amount of titanium dioxide (TiO2) particles added to RTV-1 improves hydrophobicity by over 30%, and reduces aging damage by 50% [6]. Molecular dynamics simulations indicate that TiO2 reorients the polymer’s methyl groups away from the particles. This reduces the diffusivities of water and HOCl, effectively shielding the SIR against damage. Ultimately, this means that coastal NCIs with embedded TiO2 particles can serve many more years.
SIR – TiO2 interactions
This research is funded and supported by the HVT Center at the University of Denver. For more information about this research, please visit HVTCenter.org or contact Maciej Kumosa at [email protected].
References:
[1] Fernando, M. A. R. M., Gubanski, S. M. (2010). Aging of silicone rubber insulators in coastal and inland tropical environment. IEEE Transactions on Dielectrics and Electrical Insulation, 17(2).
[2] Teverovsky A. (1999) Chlorine contamination diffusion in silicones (EEE LINKS, NASA)
[3] Amin M, Salman M (2006) Aging of polymeric insulators (an overview). Reviews on Advanced Material Science 2:93-116.
[4] Bleszynski, M., and Kumosa, M., "Aging resistant TiO2/silicone rubber composites." Composites Science and Technology 164 (2018): 74-81.
[5] Bleszynski, M., Kumosa, M. "Silicone rubber aging in electrolyzed aqueous salt environments." Polymer Degradation and Stability 146 (2017): 61-68.
[6] Bleszynski, M., Kumosa, M. "Silicone rubber RTV-1 aging in the presence of aqueous salt." IEEE Transactions on Dielectrics and Electrical Insulation 23.5 (2016): 2822-2829.
[7] Allen, Bruce, et al. "Investigation into the effects of environmental stresses on RTV-1 silicone-based caulk materials." IEEE Transactions on Dielectrics and Electrical Insulation 22.5 (2015): 2978-2986.